Researchers at the Institute for Molecular Science (IMS) have settled a two-decade-long debate about the spin direction of electrons on the surface of gold. The study was published in the Journal of the Physical Society of Japan (JPSJ).
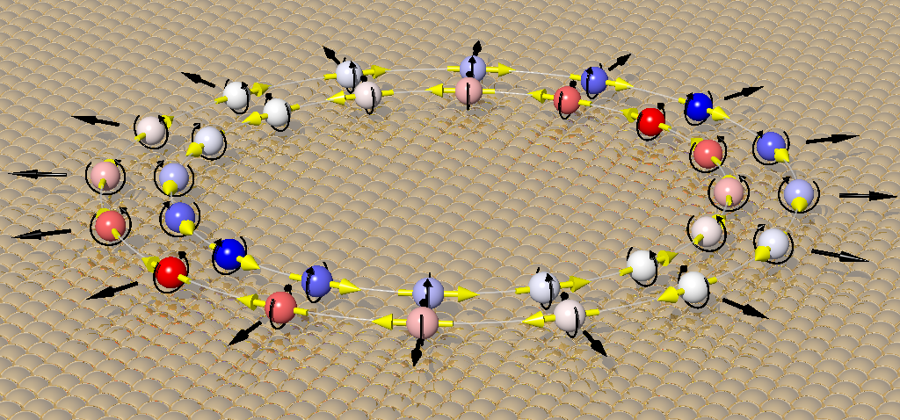 The behavior of electrons on a single-crystal gold surface was investigated. On the Au(111) surface, a difference in the magnitude of the electrons' in-plane momentum component (proportional to the in-plane velocity) is observed, which depends on their spin orientation. The two opposite assignments for the orientation of the spin were left unresolved for 20 years. Ultimately, the spin orientation and the shape of the electron's orbit were determined by measuring the two-dimensional photoelectron momentum distribution. Image Credit: Fumihiko Matsui
The behavior of electrons on a single-crystal gold surface was investigated. On the Au(111) surface, a difference in the magnitude of the electrons' in-plane momentum component (proportional to the in-plane velocity) is observed, which depends on their spin orientation. The two opposite assignments for the orientation of the spin were left unresolved for 20 years. Ultimately, the spin orientation and the shape of the electron's orbit were determined by measuring the two-dimensional photoelectron momentum distribution. Image Credit: Fumihiko Matsui
Utilizing a cutting-edge Photoelectron Momentum Microscope (PMM) at the UVSOR synchrotron facility, the team obtained comprehensive two-dimensional images of the Au(111) Shockley surface state. This allowed them to map both the electron’s spin, its intrinsic magnetic property, and its orbital shape through a projection-based measurement.
The experiment conclusively proved the Rashba effect, in which an electron's motion is connected to its spin, by assigning a clockwise (cw) spin texture to the outer electron band and a counterclockwise (ccw) texture to the inner band when observed from the vacuum side. This study creates a reliable reference dataset for spin-resolved photoemission, opening the path for the creation of extremely efficient spintronic devices.
On the surface of noble metals like gold, electrons are confined to the topmost atomic layers, forming a unique quantum state called the “Shockley surface state.” A strong electric field is produced perpendicular to the surface because it disrupts the symmetry of the crystal.
This field triggers the Rashba effect, which locks the electron's spin direction perpendicular to its motion, splitting the electronic state into two rings of electrons with opposite in-plane spin orientations.
Previous studies on ring spin direction have yielded conflicting results, with reports disagreeing on which spin is clockwise (cw) and which is counterclockwise (ccw). These discrepancies stem from variations in experimental setups, coordinate systems, and analysis conventions. To resolve this fundamental ambiguity, the IMS team employed a refined, high-efficiency imaging technique designed to overcome the limitations of prior methods.
The experiments were conducted using a twin-hemispherical-analyzer PMM at the UVSOR synchrotron facility. This specialized instrument can capture a broad, two-dimensional map of both electron momentum and energy simultaneously. To measure spin, the microscope incorporates a Spin Rotator positioned before a 2D Spin Filter (an Ir(001) crystal). The Spin Rotator is crucial because it allows for the acquisition of two images with opposing spin sensitivity without sample movement, facilitating rapid, sign-calibrated mapping.
To ensure the precise identification of electron spin polarization and its direction, the system's calibration was verified using a ferromagnetic Ni(110) reference sample. This confirmed that the detected spin signal accurately reflects the absolute physical orientation (majority/up or minority/down).
The wide-field difference images, emphasizing the contrast between electrons with opposing spins, definitively validated the previously debated spin assignment: the outer electron band exhibited clockwise (cw) rotation, while the inner band rotated counter-clockwise (ccw), as observed from the vacuum perspective.
By shining s-polarized VUV light perpendicularly onto the surface, the researchers determined that the 6s and 6p atomic orbitals primarily form the surface state. This orbital identification was confirmed by an orbital selection rule: photoelectron intensity vanished when electrons were emitted perpendicular to the light's electric field. This result experimentally shows how an electron's orbital shape (its quantum symmetry) governs its interaction with light polarization, thus demonstrating a fundamental electronic property of the surface state.
This study offers a reliable quantum standard for future materials science by definitively mapping spin and orbital textures using images. The improved PMM method enables rapid, simultaneous, and accurate signed mapping of complete 2D spin and orbital textures. In particular, the use of normal-incidence, polarization-controlled VUV light provides a novel, straightforward, and direct way to determine orbital character, aiding researchers in distinguishing genuine electronic properties from experimental errors.
With future applications in mind, this efficient method can be expanded to create a comprehensive "atlas" of spin textures across diverse materials and environments. This atlas will be crucial for the design and advancement of spintronics, a future technology that will leverage the unique properties of electron spin to create innovative functional devices.
Journal Reference:
Matsui, F., et al. (2025) Spin and Orbital Polarizations of Au(111) Surface State Determined by Photoelectron Momentum Microscope. Journal of the Physical Society of Japan (JPSJ). DOI: 10.7566/JPSJ.94.114707. https://journals.jps.jp/doi/10.7566/JPSJ.94.114707.