According to a study published in Astronomy and Astrophysics, researchers from Max-Planck-Institut für Kernphysik (MPIK) in Heidelberg have uncovered fresh information on the reaction pathways of the first molecule in space.
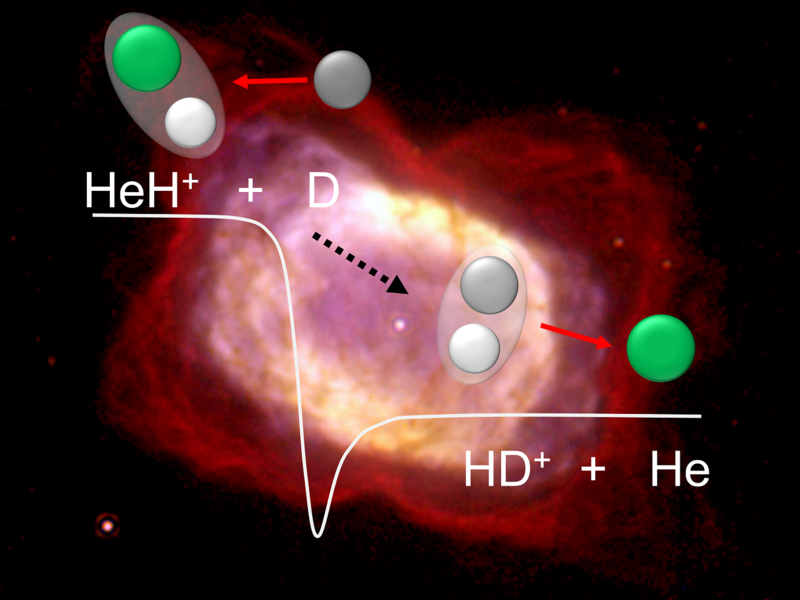
Reaction scheme and energetic level of the investigated reaction of the helium hydride ion with deuterium. It is a swift and barrierless reaction, contrary to earlier theories. Background: The planetary nebula NGC 7027, with molecular hydrogen visible in red. Copyright: MPIK; Background Image Credit: W. B. Latter (SIRTF Science Center/Caltech) and NASA
Following the Big Bang, the universe was dominated by impossibly high temperatures and density. However, within only a few seconds, it had cooled enough to allow the first elements to form, primarily hydrogen and helium.
These were still entirely ionised at this stage, as it took over 380,000 years for the universe's temperature to decrease sufficiently to allow neutral atoms to form via recombination with free electrons. This prepared the path for the initial chemical reactions.
The helium hydride ion (HeH+), which is composed of a neutral helium atom and an ionized hydrogen nucleus, is the oldest molecule known to exist. This ion is the beginning of a chain reaction that produces molecular hydrogen (H₂), the most abundant molecule in the universe.
Recombination was followed by what's known as the "dark period" of cosmology. Although the universe had become transparent thanks to free electrons binding with nuclei, there were still no light-emitting objects, such as stars. It would take several hundred million years before the first stars began to form.
However, fundamental molecules like HeH⁺ and H₂ were crucial to the development of the first stars during this early stage of the cosmos. Heat must be released for a protostar's contracting gas cloud to collapse to the point where nuclear fusion can start.
This happens as a result of collisions that excite molecules and atoms, which then release this energy as photons. However, this method loses its effectiveness for the dominating hydrogen atoms below around 10,000 degrees Celsius.
Only molecules that have the ability to rotate and vibrate to release more energy can cause further cooling. The HeH⁺ ion has long been seen as a potentially significant contender for cooling in the birth of the earliest stars because of its strong dipole moment, which makes it especially efficient at these low temperatures. Therefore, the efficiency of early star formation may be strongly affected by the helium hydride ion concentration in the cosmos.
HeH⁺ was mostly degraded during this time by collisions with free hydrogen atoms, which produced a H₂⁺ ion and a neutral helium atom. These then created molecular hydrogen by reacting with another H atom to produce a neutral H₂ molecule and a proton.
For the first time, scientists have successfully replicated this reaction under early universe-like circumstances. They looked at how HeH⁺ reacted with deuterium, a form of hydrogen that has a proton and an extra neutron in its atomic nucleus. Along with the neutral helium atom, HeH⁺ combines with deuterium to generate an HD⁺ ion rather than H₂⁺.
The experiment was conducted at the MPIK at Heidelberg's Cryogenic Storage Ring (CSR), a unique device for the study of atomic and molecular processes in a spacelike environment.
A beam of neutral deuterium atoms was placed on top of HeH⁺ ions, which were kept in the 35-meter-circumference ion storage ring for up to 60 seconds at a few kelvins (–267 °C). The researchers were able to examine the relationship between collision rate and collision energy, which is directly connected to temperature, by altering the relative speeds of the two particle beams.
They discovered that, contrary to previous predictions, the rate at which this reaction occurs does not slow with decreasing temperature, but rather remains almost constant.
Previous theories predicted a significant decrease in the reaction probability at low temperatures, but we were unable to verify this in either the experiment or new theoretical calculations by our colleagues. The reactions of HeH⁺ with neutral hydrogen and deuterium therefore, appear to have been far more important for chemistry in the early universe than previously assumed.
Dr. Holger Kreckel, Max-Planck-Institut für Kernphysik
This discovery is compatible with that of a group of theoretical physicists led by Yohann Scribano, who discovered an error in the calculation of the potential surface utilized in all prior calculations for this reaction. The updated calculations utilizing the modified potential surface now closely match the CSR experiment.
This finding sheds light on how the first stars formed, since the concentrations of molecules like HeH⁺ and molecular hydrogen (H₂ or HD) had a significant influence.
Journal Reference:
Grussie, F. et al. (2025) Experimental confirmation of barrierless reactions between HeH+ and deuterium atoms suggests a lower abundance of the first molecules at very high redshifts. Astronomy and Astrophysics. doi.org/10.1051/0004-6361/202555316.