Wave-particle duality is a fundamental and counterintuitive principle in quantum physics, describing how quantum entities exhibit both wave-like and particle-like properties.
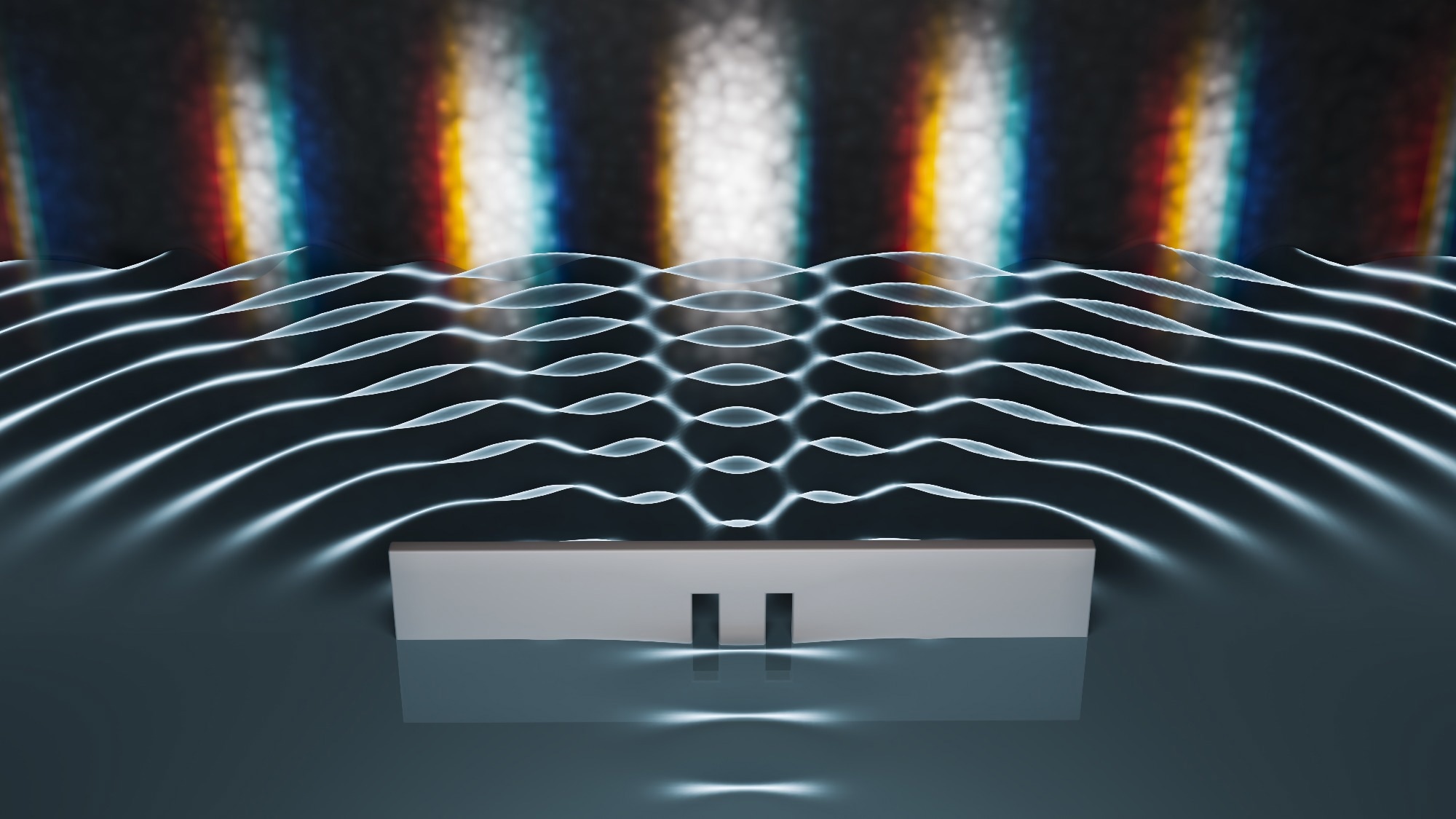
Image Credit: vchal/Shutterstock.com
While classical physics separated natural phenomena into distinct wave and particle categories (light as waves and matter as particles) quantum behavior transcends these distinctions, revealing that quantum entities follow principles that defy classical intuition.
This duality established the foundation of modern quantum physics, enabling a deeper understanding of matter and energy at microscopic scales.
Historical Context and Key Experiments
In 1678, Christiaan Huygens introduced a wave theory of light, proposing that wavefronts consist of spherical wavelets that propagate outward. This model effectively explained optical phenomena such as interference, reflection, and refraction. However, it failed to account for polarization and diffraction.
In contrast, Isaac Newton presented a corpuscular theory in 1704, describing light as discrete particles or rays. Through experiments with prisms and lenses, he demonstrated that white light comprises various colors, each with unique refractive properties. Newton's particle-based model remained dominant throughout the 18th century.
In the early 19th century, Thomas Young's double-slit experiment provided decisive evidence for the wave nature of light. The formation of interference fringes from monochromatic light demonstrated wave superposition, reinforcing Huygens' model and linking wavelength to color perception. Subsequently, James Clerk Maxwell's electromagnetic theory unified light with oscillating electric and magnetic fields, providing a comprehensive theoretical framework that confirmed its wave nature.
However, classical electromagnetism did not resolve all anomalies. The beginning of the 20th century marked the emergence of quantum theory, particularly through Albert Einstein's 1905 analysis of the photoelectric effect. Einstein's photoelectric effect study showed that light interacts with matter as discrete energy packets (now called photons) whose energy depends on frequency, not intensity. This particle-like behavior, despite light's wave propagation, revealed its dual nature.
In 1924, Louis de Broglie extended the duality to matter, proposing that particles such as electrons exhibit wave-like properties, laying the groundwork for quantum mechanics and redefining the fundamental nature of both radiation and matter.1,2
Download your PDF copy now!
Wave Behavior of Matter
Louis de Broglie introduced the concept of matter waves by extending wave-particle duality to material particles. He combined Einstein's mass-energy equivalence equation, E=mc2, with Planck's quantum relation, E=hν (where E is energy, m is mass, c is the speed of light, h is Planck's constant, and ν is frequency). By equating these expressions and substituting λ=c/ν, he derived the momentum expression p=h/λ, linking momentum to wavelength. This formulation established the theoretical foundation for wave-like behavior in particles.
De Broglie generalized the expression to λ=h/p=h/(mv), where m is the mass of the particle and v is its velocity. This formulation, known as the de Broglie wavelength, implied that all matter exhibits wave-like behavior under appropriate conditions. He introduced the term "matter waves" to describe this duality, arguing that if waves such as light can manifest particle characteristics, then particles must similarly possess wave-like features.
The Davisson–Germer experiment in 1927 provided empirical validation of de Broglie's hypothesis. By directing electrons onto a crystalline nickel target, diffraction patterns consistent with wave behavior were observed. The measured wavelengths corresponded precisely to de Broglie's theoretical predictions, thereby confirming the electron's wave properties.
Electron diffraction subsequently became a fundamental technique in materials science and nanotechnology, enabling atomic-scale imaging that has transformed disciplines ranging from biology to materials engineering.3
Particle Nature of Light and Matter
The concept of quantized energy redefined the understanding of electromagnetic radiation by demonstrating that energy is transferred in discrete units. According to quantum theory, light is composed of individual energy packets called photons, each carrying energy proportional to its frequency (E=hν). This quantization resolved key limitations of classical theories, particularly in explaining blackbody radiation and atomic emission spectra, by showing that energy exchange between matter and radiation occurs in discrete amounts, not continuously.
Einstein's 1905 interpretation of the photoelectric effect provided experimental validation for this quantization. Classical wave theory could not explain why electron emission from a metal surface occurred only when the incident light exceeded a specific threshold frequency, regardless of its intensity, nor why the kinetic energy of the emitted electrons increased with light frequency rather than amplitude. However, Einstein proposed that photons transfer their energy directly to electrons, and when this energy surpasses the metal's work function (ϕ), electrons are emitted with kinetic energy given by Kmax=hν−ϕ. This theoretical model was validated by Robert Millikan's experiments in 1914, eventually earning Einstein the 1921 Nobel Prize in Physics.4
Modern detection systems provide further evidence for the particle-like behavior of light and matter by registering individual quantum events. Photodetectors such as photomultiplier tubes and avalanche photodiodes register single photons through discrete electrical signals generated by photoelectrons. At low intensities, photons arrive individually, and each generates a distinct, measurable pulse.
Similarly, particle detection in electron and neutron diffraction experiments records single impacts, which collectively form interference patterns over time. These observations reinforce the view that quantum objects exhibit both particle and wave characteristics depending on the method of observation.
Implications for Modern Physics and Technology
Wave-particle duality plays a foundational role in various modern technologies. In digital imaging systems such as smartphones and cameras, the photoelectric effect enables image capture by allowing photons to interact with CCD or CMOS sensors. These interactions liberate electrons, generating electrical signals that form digital images, illustrating the particle nature of light.
Semiconductor technology also depends on wave-particle duality. The wave nature of electrons explains energy band structures in solids, while quantum tunneling allows charge carriers to traverse barriers forbidden by classical physics. This phenomenon supports devices like tunnel diodes, flash memory, and high-electron-mobility transistors, which are essential for high-speed computing and communication.
Scanning tunneling microscopy (STM) directly applies quantum tunneling and electron wavefunctions to image surfaces at atomic resolution. By measuring tunneling currents between a probe and sample, STM enables nanoscale imaging and manipulation, with widespread use in materials science and nanotechnology.
Quantum computing depends on superposition, a wave-derived property, enabling qubits to represent multiple states simultaneously. This capacity allows parallel processing beyond classical limits. Commercial quantum systems, such as those developed by IBM and Google, are advancing applications in cryptography, simulation, and optimization.5,6
Philosophical and Theoretical Significance
The observer effect is a challenging implication of wave-particle duality, showing that quantum systems remain in superposition until measured, at which point observation alters the system by determining a specific outcome. This results in wavefunction collapse, where a quantum system transitions from a probabilistic combination of states to a single, definite state upon measurement.
Multiple interpretations have been proposed to explain this behavior. The Copenhagen interpretation, formulated by Bohr and Heisenberg, treats wave and particle aspects as complementary and mutually exclusive descriptions necessary to explain quantum phenomena. In contrast, the Many Worlds interpretation suggests that all possible outcomes occur simultaneously in separate, parallel branches of the universe.
These interpretations highlight unresolved questions in the foundations of quantum mechanics. While the predictive success of quantum mechanics is widely accepted, debates over the role of measurement, the nature of quantum states, and the limits of objectivity continue to shape foundational research and theoretical development.7
Future Perspectives in Research and Application
Wave-particle duality remains a subject of active research, with many of its implications still in the early stages of technological exploration.
Advancements in quantum technologies will increasingly exploit wave-particle duality to enhance sensing, communication, and computation capabilities. Improved control over quantum systems is expected to reveal new physical phenomena and enable novel applications. Continued research into quantum behavior will deepen the understanding of fundamental physics while driving innovation across various scientific and technological fields.
References and Further Reading
- Siegel, E. (2024). The surprising origins of wave-particle duality. Big Think. https://bigthink.com/starts-with-a-bang/surprising-origins-wave-particle-duality/
- García López, Á. (2022). The Electrodynamic Origin of the Wave-Particle Duality. In: Banerjee, S., Saha, A. (eds) Nonlinear Dynamics and Applications. Springer Proceedings in Complexity. Springer, Cham. https://doi.org/10.1007/978-3-030-99792-2_88
- Chelsea Wald. (2025). Quantum Milestones, 1927: Electrons Act Like Waves. https://physics.aps.org/articles/v18/23
- Michael Fowler, Mark Tuckerma, et., al. (2019). Photoelectric Effect Explained with Quantum Hypothesis. https://chem.libretexts.org/Courses/BethuneCookman_University/B-CU%3ACH-331_Physical_Chemistry_I/CH-331_Text/CH-331_Text/01%3A_The_Dawn_of_the_Quantum_Theory/1.3%3A_Photoelectric_Effect_Explained_with_Quantum_Hypothesis
- Schleich, W.P. (2016). Wave-Particle Dualism in Action. In: Al-Amri, M., El-Gomati, M., Zubairy, M. (eds) Optics in Our Time. Springer, Cham. https://doi.org/10.1007/978-3-319-31903-2_19
- Matera, M. (2024). Wave-Particle Duality and its Implications for Understanding Light and Matter Interactions. Journal of Research and Development, 12(3), 1–2. https://doi.org/10.35248/2311-3278.24.12.271
- Ladj, R., Bensiradj, N., & Aid, S. E. (2024). The Many-Worlds Interpretation versus the Copenhagen Interpretation: A Case Discussion with the Hydrogen Atom. Jordan Journal of Physics, 17(4), 403-410. https://jjp.yu.edu.jo/index.php/jjp/article/view/479
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.