Mar 20 2019
From the time when superconducting mercury was first reported over a hundred years ago, the pursuit for a high-Tc superconductor has turned out to be a hot topic in physics. It was predicted that dense hydrogen would metalize and transform into a superconductor at room temperature and high pressure.
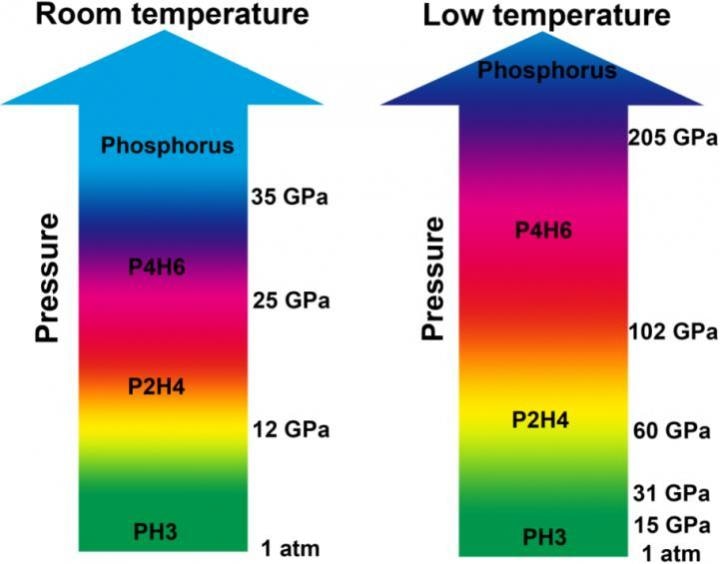 The high-pressure phase diagrams of PH3 at room temperature and low temperature. (Image credit: Science China Press)
The high-pressure phase diagrams of PH3 at room temperature and low temperature. (Image credit: Science China Press)
But it has been extremely difficult and no widely recognized experimental study has been reported to date. In 2004, Ashcroft proposed that hydrogen-dominant hydrides could turn into a high-Tc superconductor at high pressure owing to the “chemical precompression.” After that, Drozdov et al. noted the superconductive transition of H2S at 155 GPa and 203 K, which broke the highest Tc record. Most recently, LaH6 was reported to demonstrate superconducting behavior at a temperature of ~260 K. Wide-scale analyses on hydrides system have been motivated by these studies.
PH3, which is a typical hydrogen-rich hydride, has gained a lot of research interest due to its superconducting transition observed at high pressure. Yet, there was no structural information provided, and the origin of the superconducting transition stays obscure. Despite that possible structures were proposed by a series of theoretical studies, the PH3 phase under compression has stayed unknown and no relevant experimental works have been reported.
In a recent article published in National Science Review, researchers from the Center for High Pressure Science and Technology Advanced Research, School of Physics and Electronic Engineering, Jiangsu Normal University, Key Laboratory of Carbon Materials of Zhejiang Province, College of Chemistry and Materials Engineering, Wenzhou University and Shanghai Institute of Applied Physics, Chinese Academy of Sciences present their outcomes on the analyses of stoichiometric evolutions of PH3 under high pressure.
PH3 was found to be stable below the pressures of 11.7 GPa, after which it starts dehydrogenating through two dimerization processes at room temperature and pressures of up to 25 GPa. Two ensuing phosphorus hydrides, P2H4 and P4H6, were experimentally analyzed and can be recovered to ambient pressure. When P4H6 was further compressed above pressures of 35 GPa, it directly decayed into elemental phosphorus.
Low temperature can have a great impact on polymerization/decomposition under high pressure, and retain P4H6 up to at least 205 GPa.
Our findings suggested that P4H6 might be responsible for superconductivity at high pressures.
Dr Lin Wang, Study Corresponding Author
In order to ascertain the probable structure of P4H6 at high pressure, structural analyses were carried out further. Theoretical calculations showed that two stable structures with space group Cmcm (<182 GPa) and C2/m (>182 GPa) were observed. Since phonon dispersion calculations of the two structures do not offer any imaginary frequencies, this confirms their dynamic stabilities. At pressures of 200 GPa, the superconducting Tc of the C2/m structure was predicted to be 67 K.
All of these findings confirmed P4H6 might be the corresponding superconductor, which is helpful for shedding light on the superconducting mechanism.
Dr Lin Wang, Study Corresponding Author
This study was mainly supported by Natural Science Foundation of China (Grant No. 11874076), National Science Associated Funding (NSAF, Grant No. U1530402), and Science Challenging Program (Grant No. JCKY2016212A501).