Apr 22 2015
The National Physical Laboratory (NPL), the UK's National Measurement Institute in collaboration with IBM and the University of Edinburgh, has used a new quantum model to reveal the molecular structure of water's liquid surface.
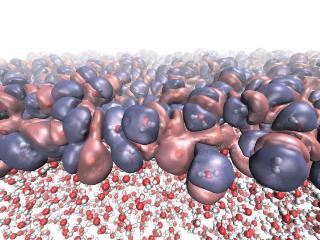 This shows the heterogeneous electronic density created by the diverse molecular orientations at the liquid-vapor interface of water. (Credit:NPL/University of Edinburgh)
This shows the heterogeneous electronic density created by the diverse molecular orientations at the liquid-vapor interface of water. (Credit:NPL/University of Edinburgh)
The liquid-vapour interface of water is one of the most common of all heterogeneous (or non-uniform) environments. Understanding its molecular structure will provide insight into complex biochemical interactions underpinning many biological processes. But experimental measurements of the molecular structure of water's surface are challenging, and currently competing models predict various different arrangements.
NPL has been working with IBM and the University of Edinburgh to make materials simulation more predictive and intuitive, by developing a new class of materials model based on quantum mechanical effects.
The model is based on a single charged particle, the quantum Drude oscillator (QDO), which mimics the way the electrons of a real water molecule fluctuate and respond to their environment. This simplified representation retains interactions not normally accessible in classical models and accurately captures the properties of liquid water.
In new research, published in a featured article in the journal Physical Chemistry Chemical Physics, the team used the QDO model to determine the molecular structure of water's liquid surface. The results provide new insight into the hydrogen-bonding topology at the interface, which is responsible for the unusually high surface tension of water.
This is the first time the QDO model of water has been applied to the liquid-vapour interface. The results enabled the researchers to identify the intrinsic asymmetry of hydrogen bonds as the mechanism responsible for the surface's molecular orientation. The model was also capable of predicting the temperature dependence of the surface tension with remarkable accuracy - to within 1 % of experimental values.
Coupled with earlier work on bulk water, this result demonstrates the exceptional transferability of the QDO approach and offers a promising new platform for molecular exploration of condensed matter.