May 8 2015
Researchers from the National Physical Laboratory (NPL), IBM and the University of Edinburgh have developed the first conceptually simple but broadly applicable model for water.
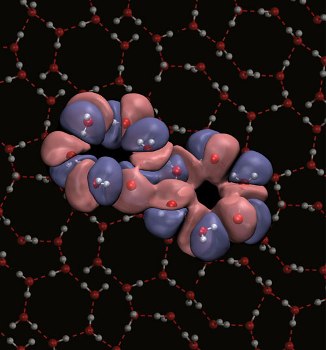 Characteristic hexagonal-ring structure of proton-ordered ice II (Image credit: NPL /University of Edinburgh)
Characteristic hexagonal-ring structure of proton-ordered ice II (Image credit: NPL /University of Edinburgh)
Water is essential to life as we know it; from its unusual density maximum which preserves freshwater ecosystems by ensuring that ponds freeze from the top down during winter, to its hydrogen bonds which shape proteins and the DNA double helix.
But while water (in its liquid, solid and vapour forms) is one of the most common substances on Earth, many of its life-sustaining properties remain a mystery. Understanding how a molecule of such apparent simplicity can encode for complex and unusual behaviour across a wide range of pressures and temperatures has been an enduring challenge for scientists.
An international collaboration involving NPL, IBM and Edinburgh University has produced a new strategy for describing matter at the atomic scale based on a simplified representation of electronic and quantum mechanical effects.
Reporting in Proceedings of the National Academy of Sciences, the team showed that their 'Quantum Drude Model' captures the full range of electronic responses of water molecules and that these are essential for a complete prediction of water's signature properties, covering liquid-gas phase equilibria from freezing to the critical point. These results establish the first conceptually simple, intuitive but broadly applicable model for water.
The method's success describing a challenging system such as water means it could be applied more generally to tackle new problems in materials science and provide insights into the molecular origin of complexity across the physical and life sciences.
Earlier this year, the researchers used their model to reveal the molecular structure of water's liquid surface in research published in the journal Physical Chemistry Chemical Physics