May 21 2018
As their name suggests, the structure of Möbius molecules is a twisted loop, a unique property with various prospective applications. A team of researchers from Japan has unearthed the characteristics of a kind of Möbius aromatic molecule that exhibits magnetism and preserves high energy levels upon exposure to light. These properties could be prospectively applied in lights, organic solar batteries, and conductive materials.
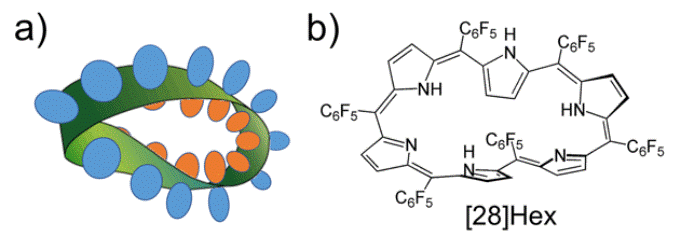 (a) The pi-electron orbit and Möbius loop in 4n organic compounds with Möbius aromatic properties. (b) Structure of the ring-shaped molecule ([28] hexaphyrin) which displays stability because of its Möbius aromatic properties (n = 7) in its ground state. (Image credit: Kobe University)
(a) The pi-electron orbit and Möbius loop in 4n organic compounds with Möbius aromatic properties. (b) Structure of the ring-shaped molecule ([28] hexaphyrin) which displays stability because of its Möbius aromatic properties (n = 7) in its ground state. (Image credit: Kobe University)
The team headed by Professor Yasuhiro Kobori (Kobe University), Professor Atsuhiro Osuka (Kyoto University), Professor Kazunobu Sato and Project Professor Takeji Takui (Osaka City University) made the findings. The research has been reported in The Journal of Physical Chemistry Letters on May 10, 2018.
The attention gained by the Möbius aromatic molecules is due to their ability to be energized by light. Upon being energized, in their electronically excited state, they exhibit “antiaromaticity,” attributed with high instability and high energy levels. Eco-friendly organic devices, such as electroluminescent elements and organic thin-film solar cells, can be developed by using this excited state. Yet, the facts behind the antiaromatic properties as well as the electronic properties of this state were obscure.
In this research, the researchers employed a time-resolved electron paramagnetic resonance technique which involves the use of electromagnets and microwaves for the detection of the magnetic characteristics of a reactive intermediate. The excited triplet state of [28] hexaphyrin, a Möbius aromatic molecule, was observed by illuminating it with laser pulses. They detected the resonance between the microwave and the electron spins associated with the excited triplet state’s magnetism and with the external magnetic field as a snapshot with a precision of 10 million parts per second after every laser pulse.
The researchers also altered the polarization angle of the laser pulse with respect to the direction of the external magnetic field, which enabled them to clarify the three-dimensional location of the triplet spin, and also to capture the deactivation process on sub-levels of the triplet at the rate of 10 million “snapshots” per second.
The investigation showed that twisted ring molecules have a “charge-transfer” property that liberates and localizes the charge at right angles between the orbitals. The charge transfer prevents the stabilizing effect brought about by the exchange interaction between the electrons, thereby resulting in higher energy to provide the source of the strong antiaromatic characteristics of the molecule.
The electron distributions in the current triplet state are very different when compared to those in the excited singlet state species which does not display magnetism. This research has revealed that every electron distribution is localized in one part of the ring framework of the molecule. It also demonstrates that when the orbital angular momentum between the localized orbitals in the triplet state is altered, one sub-level is quickly deactivated to the ground state.
Such orthogonal orbital angle relations occur only in the twisted Möbius topology, indicating that the deactivation could open the door for innovative tools that can be used for indexing antiaromatic properties and for studying the excited state geometry.
The special electronic properties of this highly active excited state could be applied in electronic functional materials, such as organic solar cells and electric conductors, and could potentially contribute to the solution of energy and environmental issues.
Professor Yasuhiro Kobori