Aug 25 2015
The combination of ab-initio numerical experiments and theory shows that optical tunnelling of an electron from an atom can occur instantaneously.
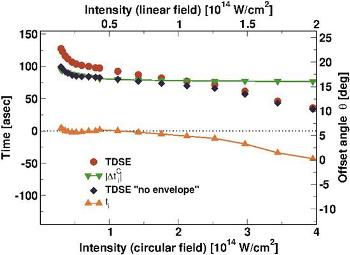 Ionization times (left axis) reconstructed using the ARM theory from offset angles (right axis) obtained numerically using TDSE calculations. Red circles are the numerically calculated offset angles, divided by the laser frequency, è/ù. Blue diamonds show the offset angles with the correction due to the substraction of the pulse envelope effect, ti0 = è/ù-|Ätienv (è,ppeak)| . Green inverted triangles show the Coulomb correction to the ionization time evaluated at the peak of the photoelectron distribution, |ÄtiC (è,ppeak|. Orange triangles show the ionization times we obtain by applying the reconstruction procedure defined by equation (4) in the paper. In terms of the figure, this is simply the result of subtracting the green curve from the blue curve. (c) MBI
Ionization times (left axis) reconstructed using the ARM theory from offset angles (right axis) obtained numerically using TDSE calculations. Red circles are the numerically calculated offset angles, divided by the laser frequency, è/ù. Blue diamonds show the offset angles with the correction due to the substraction of the pulse envelope effect, ti0 = è/ù-|Ätienv (è,ppeak)| . Green inverted triangles show the Coulomb correction to the ionization time evaluated at the peak of the photoelectron distribution, |ÄtiC (è,ppeak|. Orange triangles show the ionization times we obtain by applying the reconstruction procedure defined by equation (4) in the paper. In terms of the figure, this is simply the result of subtracting the green curve from the blue curve. (c) MBI
How long does it take an atom to absorb a photon and loose an electron? And what if not one but many photons are needed for ionization? How much time would absorption of many photons take? These questions lie at the core of attosecond spectroscopy, which aims to resolve electronic motion at its natural time scale.
Ionization in strong infrared fields is often viewed as electron tunnelling through a potential barrier, created by the combination of the atomic potential that binds the electron and the electric field of the laser pulse that pulls the electron away. Thus, unexpectedly, attosecond spectroscopy finds itself facing an almost age-old and controversial question: how long does it take an electron to tunnel through a barrier?
In the paper by Torlina et al, this question is studied by using the so-called atto-clock setup. The attoclock uses the rotating electric field of a circularly polarized laser pulse as a hand of the clock. One full revolution of this hand takes one laser cycle, about 2.6 fs for experiments with 800 nm pulse of a Ti-sapph laser. As the electric field rotates, so does the tunnelling barrier. Thus, electrons tunnelling at different times will tunnel in different directions. This link between time and direction of electron motion is what allows the attoclock to measure times. In every clock, a time zero must be established. In the attoclock, this is done by using a very short laser pulse, which lasts only one-two cycles. Tunnelling occurs in a small window where the rotating electric field passes through its maximum.
Next, like any other clock, the attoclock must be calibrated. One has to know how the time of electron emission – its exit from the tunnelling barrier – maps onto the angle at which the electron is detected. This calibration of the attoclock has now been accomplished by Torlina et al, with no ad-hoc assumptions about the nature of the ionization process or the underlying physical picture.
Combining analytical theory with accurate numerical experiments, and having calibrated the attoclock, the authors could finally carefully look at delays in electron tunnelling. They arrive to the surprising answer: this time delay may be equal to zero. At least within the realm of non-relativistic quantum mechanics, the electron tunnelling out of the ground state of a Hydrogen atom spends zero time under the tunnelling barrier. The situation may change, however, if this electron encounters other electrons on the way, which may become important in other atoms or molecules. The interaction between the electrons may lead to delays.
Thus, the attoclock provides a unique window not only into the tunnelling dynamics, but also into the interplay of different electrons that participate in the ionization process, and how the electrons staying behind readjust to the loss of their comrade.