Feb 19 2021
Under the guidance of Professor Stephan Schiller, PhD, from Heinrich Heine University Düsseldorf (HHU), a working group has utilized an innovative, high-precision laser spectroscopic experiment to quantify the internal vibration of the simplest molecule.
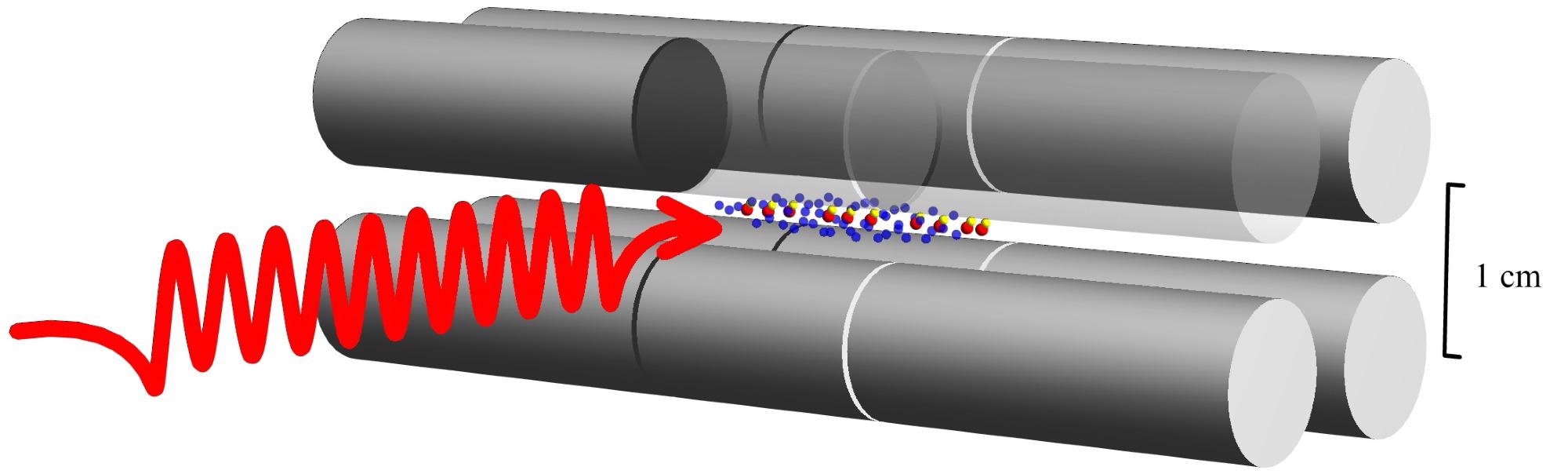 HD+ molecular ions (yellow and red dot pairs) in an ion trap (grey) are irradiated by a laser wave (red). This causes quantum jumps, whereby the vibrational state of the molecular ions changes. Image Credit: Heinrich-Heine University Düsseldorf /Soroosh Alighanbari.
HD+ molecular ions (yellow and red dot pairs) in an ion trap (grey) are irradiated by a laser wave (red). This causes quantum jumps, whereby the vibrational state of the molecular ions changes. Image Credit: Heinrich-Heine University Düsseldorf /Soroosh Alighanbari.
This enabled the group to examine the wave character of the motion of atomic nuclei with unparalleled precision. The study results have been published in the current edition of the Nature Physics journal.
Nearly 10 decades ago, a revolutionary breakthrough was achieved in the field of physics: microscopic matter was found to have wave properties.
For several decades, increasingly accurate experiments have been employed to quantify the wave properties of electrons specifically. Such experiments significantly relied on spectroscopic analysis of the hydrogen atom and they allowed confirming the precision of the quantum theory of the electron.
For bulky elementary particles, like protons, and nuclides (atomic nuclei), it is hard to quantify their wave properties precisely. But theoretically, such properties can be observed everywhere.
The wave properties of atomic nuclei in molecules are clear and can be noted in the internal vibrations of the atomic nuclei opposed to each other.
These vibrations are allowed by the electrons in molecules, which make a bond between the nuclei that is 'soft' instead of rigid. For instance, nuclear vibrations arise in every molecular gas under normal conditions, for example, in the air.
The atomic nuclei’s wave properties are shown by the fact that the vibration cannot have an arbitrary strength, or energy, similar to a pendulum for instance. Rather, only accurate, discrete values called 'quantized' values are feasible for the energy.
A quantum jump from the least vibrational energy state to a greater energy state can be realized by irradiating light onto the molecule, whose wavelength is set accurately so that it matches precisely the energy difference present between the two states.
For a very precise analysis of the wave properties of nuclides, one would require both a very accurate measuring technique and a very accurate knowledge of the binding forces in the particular molecule, since these govern the details of the nuclides’ wave motion.
Then, this enables testing of the basic laws of nature by comparing their particular statements for the nuclide examined with the measurement outcomes.
However, generally, accurate theoretical predictions of the binding forces of molecules are not possible yet—the quantum theory to be employed is too hard to handle mathematically.
As a result, it is not feasible to precisely examine the wave properties in any specified molecule. This can only be realized with specifically simple molecules.
The research group of Professor Schiller is dedicated to exactly one such molecule, specifically the hydrogen molecular ion HD+ that consists of a nuclide deuteron (d) and proton (p). This was done with the group’s long-standing collaboration partner V. I. Korobov from the Bogoliubov Laboratory of Theoretical Physics at the Joint Institute for Nuclear Research in Dubna, Russia.
The deuteron and proton are connected by a single electron. The comparatively simple nature of this molecule implies that extremely precise theoretical calculations can now be carried out. V.I. Korobov achieved this by improving his calculations constantly for more than two decades.
In the case of charged molecules like the hydrogen molecule, an accessible yet highly accurate measuring method was not available to date.
But in 2020, the team under the guidance of Professor Schiller created an innovative spectroscopy method for examining the rotation of molecular ions. The radiation utilized at that time is known as 'terahertz radiation,' with a wavelength measuring around 0.2 mm.
Currently, the team could demonstrate that the same method also works for the excitation of molecular vibrations through irradiation with a 50 times shorter wavelength. The researchers performed by designing a specifically frequency-sharp laser that is one of a kind globally.
They showed that this method of expanded spectroscopy has a resolution capacity for the radiation wavelength for vibrational excitation 10,000 times higher compared to earlier methods employed for molecular ions.
Systematic disturbances of the vibrational states of the molecular ions, for instance, by interfering magnetic and electrical fields, could also be inhibited by a factor of 400.
Eventually, it appeared that the forecast of quantum theory concerning the behavior of the atomic nuclei deuteron and proton matched with the experiment with a comparable imprecision of below 3 parts in 100 billion parts.
If it is supposed that the forecast of V.I. Korobov based on quantum theory is complete, the outcome of the experiment can also be differently deciphered—especially as the determination of the ratio of electron mass to proton mass.
The value derived matches very well with the values established through experiments by other research groups with the help of totally different measurement methods.
We were surprised at how well the experiment worked. And we believe that the technology we developed is applicable not only to our ‘special’ molecule but also in a much wider context. It will be exciting to see how quickly the technology is adopted by other working groups.
Stephan Schiller, Professor, Heinrich Heine University Düsseldorf
Journal Reference
Kortunov, I. V., et al. (2021) Proton–electron mass ratio by high-resolution optical spectroscopy of ion ensembles in the resolved-carrier regime. Nature Physics. doi.org/10.1038/s41567-020-01150-7.