Sep 15 2015
One of the first things taught in school science classes is that there are three states of matter - solids, liquids and gases. Bizarrely, however, at high pressures and temperatures there is a critical point above which the distinction between a liquid and a gas is lost and a single 'supercritical fluid' is formed.
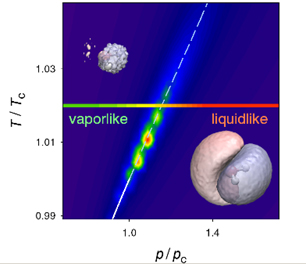 Widom line and the variation of molecular dipole moment between vapour-like and liquid-like domains, with corresponding electron densities
Widom line and the variation of molecular dipole moment between vapour-like and liquid-like domains, with corresponding electron densities
Here, thermodynamics says, there are no phase boundaries, creating a continuous path from liquid to gas and widely tuneable properties which make supercritical fluids promising green alternatives to chemical solvents.
For the first time, an international team comprising the National Physical Laboratory (NPL), the University of Edinburgh and IBM Research has discovered that a primitive form of the liquid-gas transition extends far into the supposedly featureless supercritical phase at the molecular scale in water and demonstrated that the conclusions are likely to apply more generally.
Reporting in Physical Review Letters, the team use a powerful new quantum mechanical model which they recently developed to identify the onset of intermolecular bonds (the formation of a primitive liquid from dissociated molecules) and electron redistribution in supercritical water.
Abrupt changes in the molecular electronic charge distribution are found to occur at the point where the bonds between molecules are first formed marking an unambiguous separation between dissociated (gas) and associated (liquid) regimes in the supercritical fluid.
The results imply that supercritical fluids exhibit far richer behaviour on the molecular scale than conventional thermodynamics predicts.
Read the full paper: Molecular-scale remnants of the liquid-gas transition in supercritical polar fluids by V. P. Sokhan, A. Jones, F. S. Cipcigan, J. Crain and G. J. Martyna in Physical Review Letters.
Contact: Vlad Sokhan