May 14 2015
The recombination of electron shells in molecules, taking just a few dozen attoseconds (a billionth of a billionth of a second), can now be viewed "live," thanks to a new method developed by MIPT researchers and their colleagues from Denmark, Japan and Switzerland. An article detailing the results of their study has been published in the journal Nature Communications.
 This shows the laser-induced electronic-structure effects. Credit: © ETH Zurich
This shows the laser-induced electronic-structure effects. Credit: © ETH Zurich
In recent years, scientists have learned how to study ultrafast processes taking place at the atomic and molecular levels, and research in this field is expected to yield some very important results. In Germany, for instance, scientists are creating the European X-Ray Free-Electron Laser (XFEL).Russia, too, is participating in the project. Once built, XFEL should give the scientists an opportunity to observe changes occurring in molecules' nuclei during chemical reactions, which matters a great deal for the study of biochemical processes and proteins' structural properties.
Two groups of scientists - experimentalists led by Professor Hans Jakob Wörnerof the Swiss Federal Institute of Technology in Zurich and theoreticians from Denmark, Japan and Russia headed by MIPT's Oleg Tolstikhin - have joined their efforts to study attophysical processes, which are processes lasting several attoseconds (10^-18 seconds).
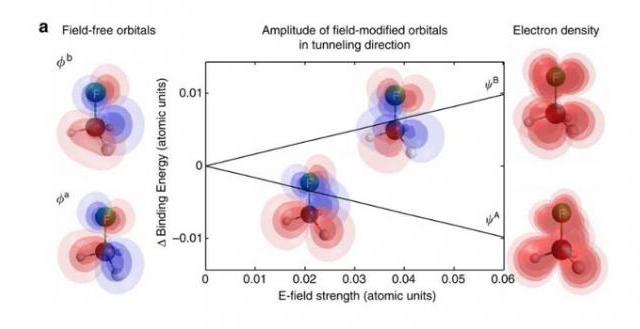
To track processes taking virtually no time to happen, the scientists used the so-called pump-probe method. First, a molecule was impulsively oriented with one laser pulse. Then a second powerful, low-frequency laser pulse ionized the molecule, which generated high harmonic radiation. By looking at the high harmonic spectrum, Wörner's group was able to see the restructuring of the molecule's electron shell caused by the ionizing pulse's strong field, which is a significant step forward for attosecond spectroscopy.
"With this method, we were able to track structural changes in the electron shells of methyl fluoride (CH3F) and methyl bromide (CH3Br)molecules," said Oleg Tolstikhin, associate professor at MIPT's Theoretical Physics Section. "These processes are even faster than chemical reactions, in which atomic nuclei move. In this experiment, we were able to see the restructuring of the electron shell."
The experimental set-up consisted of a sapphire laser with a wavelength of 800 nanometers, which generated short pulses of very high intensity (10^14-10^15 watts per cm2). The amplitude of the electromagnetic field in such pulses is comparable to that in an electric field, which "feels" the electron in a hydrogen atom. The laser hit its targets - methyl fluoride and methyl bromide gas molecules in a vacuum chamber. The researchers then analyzed the spectrum of the generated high harmonics using X-ray and ultraviolet spectrometers.
"This was the first time ever that the evidence of the restructuring of a molecule's electron shell caused by its interaction with the strong field of an ionizing laser pulse was observed in the high harmonic spectrum," said Tolstikhin. "The observed processes lasted a few tens of attoseconds. Identifying the traces of such processes in high harmonic spectra was possible thanks to our asymptotic theory of the tunneling ionization of molecules in the case of degenerate electronic states. Our theoretical model describes the experimental results pretty well."
Tolstikhin also explained that the scientists were unable, and are unlikely to ever be able, to see moving electrons -that's ruled out by the laws of quantum mechanics. But what they did see is how the electron cloud "migrated" within the molecule. A key role in such "migration" is played by a permanent dipole moment and degenerate states of the outer electron in the molecule. This was the reason why the researchers chose methyl fluoride and methyl bromide molecules for their study.
The method of tracking attoseconds-long processes, demonstrated in the experiment, opens up new possibilities for studyingfine chemical processes, which can be of critical importance for molecular biology.