The magic isotope tin-100 has proved challenging for physicists to investigate. New research may have put scientists on the right track.
Image Credit: zizou7/Shutterstock.com
New research sheds light on the so-called “doubly magic” atomic nuclei of the tin isotope, tin-100.
Atomic nuclei may appear fairly simple—after all, every element has a nucleus made up of just two components, protons and neutrons. Tin-100 is the heaviest known nucleus, with an equal number of protons and neutrons : 50 protons and 50 neutrons.
It is when the number of these constituents — collectively called nucleons — are changed that things start to get interesting.
In a paper published in the journal Nature Physics, CERN researcher Maxime Mougeot and her co-authors describe both theoretical calculations and experimental results from CERN’s ISOLDE facility that could shed new light on tin-100, one of the most interesting isotopes of any element.
Protons, and Neutrons, and Nucleons… Oh My!
Every element is defined by the number of protons it contains, however there is a little more flexibility with neutrons. An atomic nucleus can contain different numbers of neutrons without becoming a different element. Elements with differing numbers of neutrons are called isotopes.
With reference to carbon, the “standard” stable isotope of carbon is carbon-12, named because with six protons and six neutrons they have 12 nucleons. As common in the atmosphere is the isotope carbon-14, with six protons and eight neutrons, 14 nucleons in total.
Carbon-14 is not stable, however, and decays in nitrogen-14. The number of nucleons has stayed the same but via the emission of beta particles — either an electron or a proton — in a process called beta decay, a neutron has changed to a proton.
That means the total protons are now seven and the neutrons number eight. The number of nucleons has not changed but the element is now nitrogen-14, not carbon-14.
Tin-100 is also remarkable because it is what is known as a “doubly magic” isotope.
Atomic Physics: It’s a Kind of Magic
In quantum physics, the “magic numbers” correspond to nuclear structures called energy shells. Every one of these shells can be filled by a certain number of particles—that number is a magic number.
Atoms have magic numbers that describe filled shells of electrons, while atomic nuclei have a different set of magic numbers corresponding to filled shells of protons and neutrons.
The magic numbers for energy shells in an atom, corresponding to shells of electrons around an atomic nucleus, are 2, 10, 18, 36, 54, and 86. Meanwhile, the magic numbers for shells of protons and neutrons in a nucleus are 2, 8, 20, 28, 50, 82, and 126.
A magic number nucleus is one with either protons or nucleons equal to one of these latter sets of numbers. Tin-100 is known as a “doubly magic” nucleus, which means that it has protons that are equal to a quantum magic number, and neutrons totaling a magic number too.
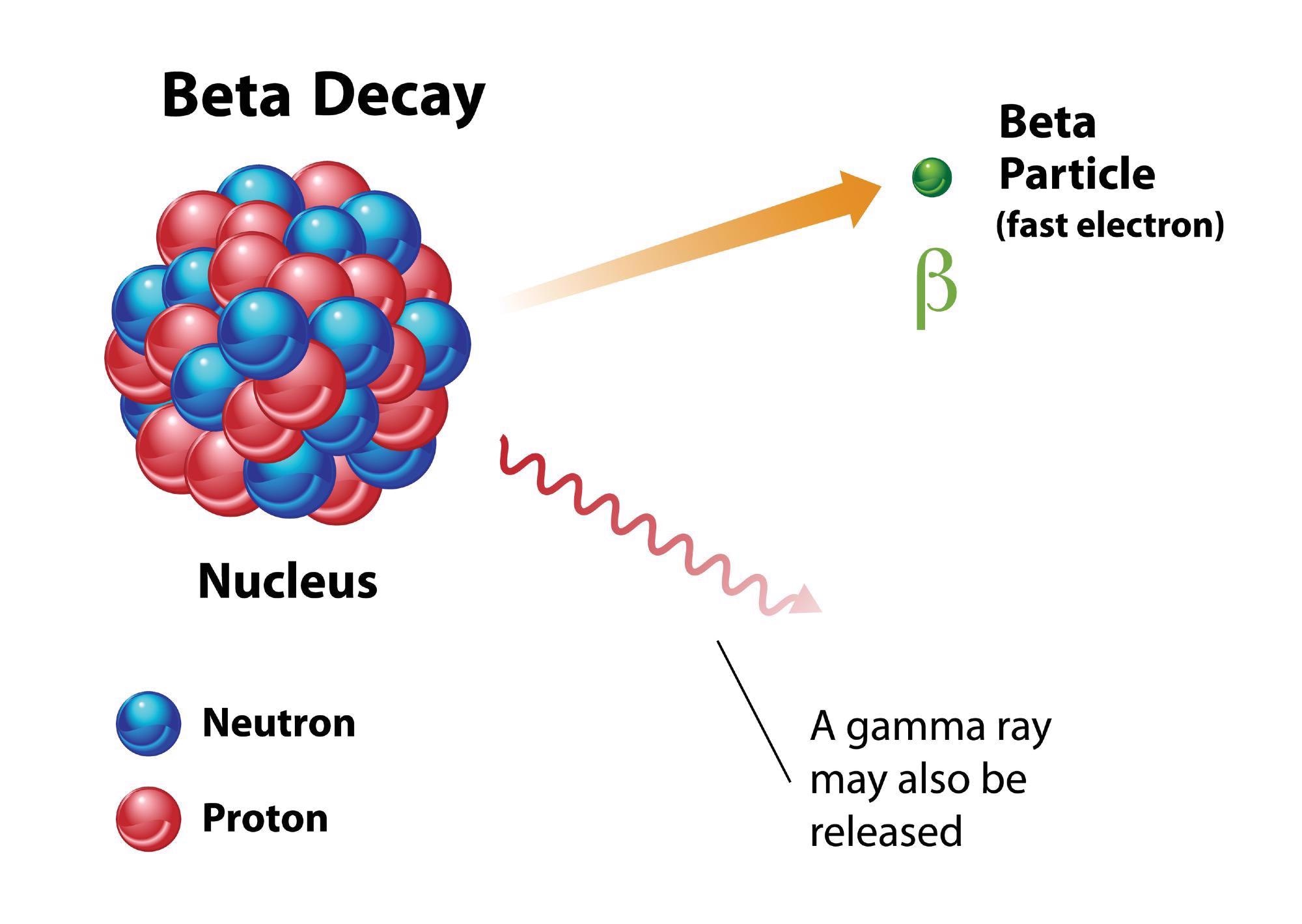
Image Credit: OSweetNature/Shutterstock.com
Stable tin comprises 112 nuclear particles — 50 protons and 62 neutrons. The neutrons act as a kind of buffer between the electrically repelling protons and prevent normal tin from decaying. According to the shell model of nuclear physics, 50 is a “magic number” that gives rise to special properties.
Tin-100, with 50 protons and 50 neutrons, is “doubly magic,” making it particularly interesting for nuclear physicists. What makes tin-100 unusual, is most isotopes with doubly magic numbers are particularly stable, but tin-100 decays fairly rapidly.
Beta-Decay in Tin-100
When researchers have attempted to produce beta decay in tin-100 they have had difficulty, and have measured varying amounts of energy being released. This has led to confusion over the mass of tin-100.
This confusion has not been helped by the fact tin-100 is an unstable isotope of tin, as mentioned above. It is also a difficult isotope to create.
Recent experiments at the Isotope Mass Separator On-Line (ISOLDE )facility —a unique source of low-energy beams of radioactive nuclides, those with too many or too few neutrons to be stable — have enabled the production of the nuclei indium-101, indium-100, and indium-99, significant because they are just one proton less than tin-100.
The authors of this new study used ISOLDE’s high-precision mass spectrometer ISOLTRAP to capture these isotopes and measure their mass, focusing particularly on indium-100.
ISOLTRAP delivers a measurement of indium-100 that is 90 times more precise than previous attempts at measurements.
They discovered that the mass of tin-100 can be derived from indium-100, and that this leads them to the energy that is released when the tin isotope decays into the isotope of indium.
The researchers then compared the measured mass of indium-100 with theoretical calculations. The result directs physicists to the correct models for tin-100.
Additionally, the comparison between measurement and theory led to an excellent level of agreement, indicating that the researchers are on the right path to unlocking the nuclear physics secrets of tin-100 and its indium neighbors.
With tin-100 being such a short-lived isotope and so difficult to produce, the isotope does not currently have too many applications. However, better understanding this isotope could help develop a more precise picture of beta decay, something that can be used in the treatment of bone cancer and certain eye conditions.
Reference
Mougeot. M., Atanasov. D., Karthein. J., et al, [2021], ‘Mass measurements of 99–101In challenge ab initio nuclear theory of the nuclide 100Sn,’ Nature Physics, [https://doi.org/10.1038/s41567-021-01326-9]
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.