Theories must stand up to practical testing, and this is especially true in physics. Researchers from Johannes Gutenberg University Mainz (JGU), Texas A&M University, Brookhaven National Laboratory, the University of Surrey in the UK and Michigan State University have achieved such a milestone: They were able to experimentally demonstrate for the first time that the ratio method can be used to study atomic nuclei, and in particular unstable halo nuclei – thus underscoring the importance of this new reaction observable.
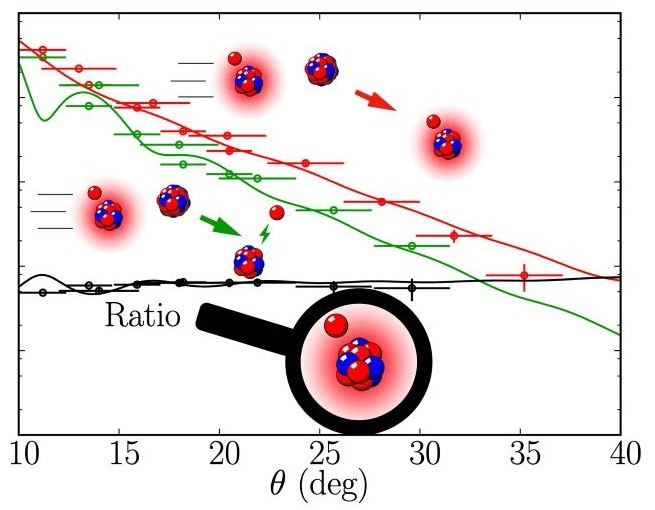 The red line shows the scattering cross section, the green line the breakup cross section. The dots correspond to the experimental data, the lines to the theoretical calculations. Image Credit: Capel, JGU
The red line shows the scattering cross section, the green line the breakup cross section. The dots correspond to the experimental data, the lines to the theoretical calculations. Image Credit: Capel, JGU
“Our studies on the beryllium-11 halo confirmed the theoretical predictions of the ratio method,” says Prof. Dr. Pierre Capel from JGU. This important result offers nuclear physicists a new tool for investigating the structure of exotic nuclei. The team published their results on May 28, 2025, in the renowned scientific journal Review Physics Letters.
Ratio Method Provides Precise Information
Halo nuclei are significantly larger than conventional atomic nuclei. This is due to a very peculiar structure in which one or two neutrons can decouple from the nucleus and form a kind of diffuse halo around a compact core. Halo nuclei also differ from most other atomic nuclei in terms of their stability: They have an extremely short half-life, in case of the beryllium-11 halo being studied it is just 13 seconds. This means that after 13 seconds only half of the halo nuclei produced still exists, while the other half has already decayed.
To study them, experimentalists collide them with a target, and the outcome of the reaction is used to draw conclusions about the structure of the nucleus. The problem is that the information about the halo nucleus is difficult to separate from influences that arise during the experiment.
In 2011, the three theorists P. Capel and R.C. Johnson of the University of Surrey and F.M. Nunes of Michigan State University developed the ratio model. “We determine the structure of halo nuclei from the ratio of their scattering and breakup cross sections—in this way, we eliminate the influence of the reaction and obtain information about the pure structure of the halo nucleus,” says Capel. The scattering cross section is the process by which the projectile is scattered from the target and remains intact after the collision, while the breakup section is the process by which the halo neutron is split off from the nucleus.
First Experimental Evidence Achieved
At Texas A&M University, the experimental team created beryllium-11 using a particle accelerator and collided them with the stable atomic nuclei of carbon-12. “We were able to show that the cross sections for scattering and breakup have very similar characteristics – their ratio is therefore independent of the reaction process. This demonstrates that the ratio method works,” says P. Capel.
In a next step, the researchers plan to investigate carbon-19, another halo nucleus. The team expects this measurement to determine the separation energy of carbon-19 more precisely than ever before and provide important information about the halo structure of carbon-19.