Feb 1 2017
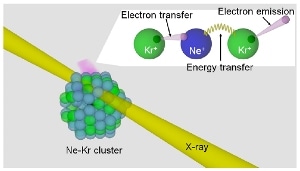 Scientists have elucidated a novel mechanism of electron emission from matter caused by x-rays. In the studied model system, X-rays produce the doubly-charged particle (Ne2+), which catches an electron from one of the neighboring atoms (Kr), transferring the energy to the other and releasing another electron. Credit: Kiyoshi Ueda
Scientists have elucidated a novel mechanism of electron emission from matter caused by x-rays. In the studied model system, X-rays produce the doubly-charged particle (Ne2+), which catches an electron from one of the neighboring atoms (Kr), transferring the energy to the other and releasing another electron. Credit: Kiyoshi Ueda
A research team headed by Kiyoshi Ueda from Tohoku University has analyzed what x-rays in matter really do and established a new mechanism to generate low-energy free electrons. Due to the fact that low-energy electrons have the potential to damage the matter, the established mechanism is expected to be highly significant in interpreting and outlining radiation treatment for various illnesses.
In biology, medicine, and the material sciences, X-rays are the most significant diagnostic tool. This is because of their ability to penetrate deep into materials that are opaque to the human eye. However, the passage of X-rays through a sample can have side effects as energy is deposed into the deep layers of the sample due to X-ray absorption.
In certain serious cases, X-ray application is restricted due to the side effects - known as “radiation damage.” One area where the dose of the absorbed X-ray has to be reduced is medicine.
Surprisingly, there is no clue related to the consequences of X-ray absorption, for instance, in biological tissue comprising of biomolecules, water, and certain metal atoms. One explanation for this phenomenon could be that the first few reaction steps after the X-ray absorption occur in a highly rapid fashion, within 10-100 femtoseconds, where one femtosecond is equal to 10-15 seconds, or conversely, one millionth of one billionth of a second.
In this short time gap, multiple electrons are emitted through a complex cascade of events, and positively charged reactive particles (ions) are generated. Most experiments performed until now could only characterize the final state that occurs a long time after the completion of the cascading reaction. Yet, the accurate knowledge related to the intermediate steps is highly significant to predict and design radiation effects in matter.
The researchers have performed an experiment that provided an unparalleled detailed view of the first few hundred femtoseconds after the absorption of X-ray by matter.
In a biological system, a lot of water molecules are found to be flexibly positioned around biologically functional molecules, but they are not strongly bound to the functional molecules.
In order to create a model biological system, a flexible, weakly bonded aggregate involving two different noble gases, Ne and Kr, was developed by cooling the gases to extremely low temperatures. Subsequently, the Ne-Kr clusters were exposed to pulsed X-rays from the SPring-8 synchrotron radiation source. Under the conditions selected for the experiment, the radiation source favorably ionized the Ne atoms.
The research team employed an advanced experimental set-up to record all of the ions and electrons generated during each X-ray absorption event. The researchers discovered that just after a few hundred femtoseconds of the initial ionization, the Ne atom that absorbed the X-ray and two neighboring Kr atoms were in a positively charged, ionized state.
The research team member Lorenz Cederbaum theoretically proposed this mechanism through which the ultrafast charge redistribution progresses. The mechanism has been termed the “Electron Transfer Mediated Decay” (ETMD), and involves transfer of electrons to the originally ionized Ne atom corresponding with energy transfer away from the Ne, resulting in the ionization of the neighboring Kr atom. It was evident from the experiment that highly localized charge generated by X-rays in matter gets redistributed over many atomic sites in an astonishingly short time gap.
We believe that understanding X-ray initiated processes on a microscopic level will lead to new insights across the disciplines of physics, biology and chemistry.
Kiyoshi Ueda, Tohoku University
The outcome of the research has been published in Nature Communications, a scientific journal.